 Mark Hammar
Mark Hammar
May 6, 2014
ISO 9001 Clause 7.6 is often quickly looked over without much thought, but what is it really all about? In a recent article, Monitoring and Measurement: The basis for evidence-based decisions, I discussed the importance of using good evidence-based decisions to improve the effectiveness and efficiency of your organization. I also mentioned that this evidence needs to be accurate and adequate. It is because of this that the equipment used needs to be controlled to ensure that the data evidence is accurate. The following article further explains what ISO 9001 says about the control of this equipment.
The first thing that is discussed in Clause 7.6 predicts the information in Clauses 8.2.3 & 8.2.4 about monitoring and measurement. What needs to be done by the organization is to:
The outcome of these first steps is that the company will have determined the why, how and when for the use of monitoring and measurement equipment.
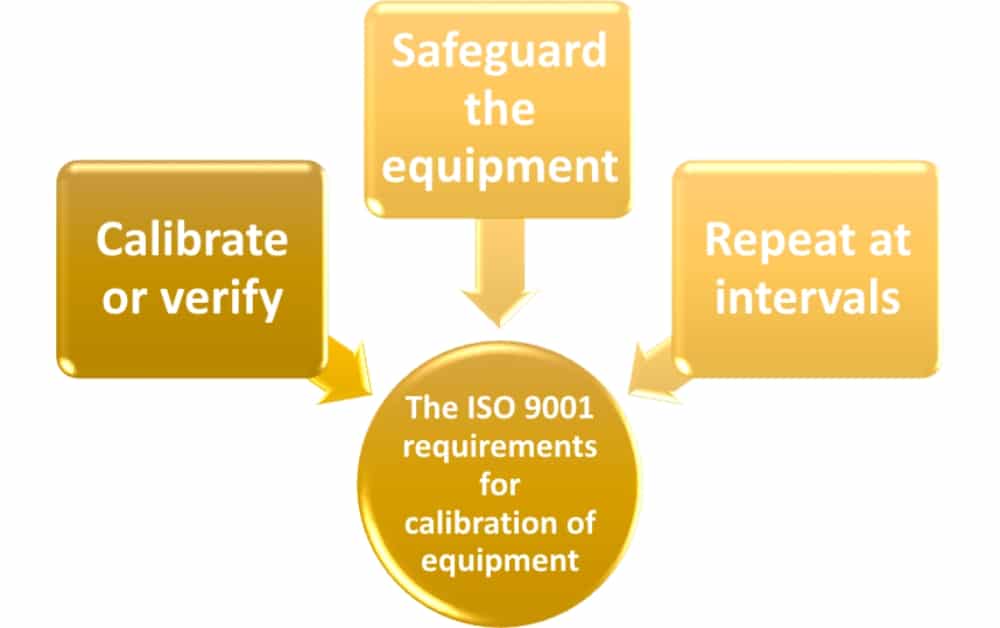
This main section, often called calibration, is often thought of as the whole purpose of Clause 7.6. This is the main reason that this clause is glossed over if the company already has a system in place for equipment calibration. The requirements for calibration of equipment are fairly simple, and follow this logic:
Calibrate or verify. This is simply using a standard to ensure that the equipment being calibrated or verified is accurate compared to the standard. The standard should be traceable to international or national standards, but where such standard does not exist you need to record what was used. Probably the most common example of this would be using a set of confirmed accurate gauge blocks to calibrate that a thickness measuring device, such as a caliper, is taking accurate measurements.
Safeguard the equipment. After being calibrated, the equipment needs to have some way to make sure that someone can’t adjust the equipment so that it no longer meets the requirements, thereby making the calibration invalid. Additionally, the equipment needs to be protected so that it does not deteriorate or become damaged during use. For example, if you are putting sensitive measurement equipment into a dirty machine shop you will need to take precautions to ensure that the equipment is not ruined by the dirt in the environment.
Repeat at intervals. Calibration needs to be done routinely, at times based upon the type of equipment (often at 3-, 6- or 12-month intervals). Part of the safeguarding is to ensure that there is some way to identify that the equipment is calibrated and when the calibration check needs to happen again. Often companies will attach a sticker showing that the equipment has been calibrated with a date indicating the next calibration due date. By doing this anyone picking up the piece of equipment can tell that it is calibrated, and if the date is in the future that the calibration does not need to be re-done yet. During these repeated calibrations, the equipment is to be adjusted or re-adjusted as necessary if the equipment accuracy needs to improve or does not meet the requirements. When it happens that the equipment is not meeting requirements, the organization needs to review any product that has been measured with that equipment. It must be decided what actions need to happen to ensure that the product did meet requirements, or to accept the product without further verification.
Of course, keeping records of all of these activities is necessary.
As part of this, it is important to remember that software which is used to monitor and measure product or services against requirements, such as software controlling an automatic measurement device, needs to have confirmation that it will satisfy the intended application. (This is not applicable to software supplied as a product.) This needs to happen before use, and if necessary, it needs to be reconfirmed. This will typically include verification of the software to show that it does what is intended and configuration management to control and track changes to the software to ensure that it maintains its suitability for use.
While it is true that a good calibration system based on requirements such as ISO 10012:2003 measurement management systems, will create the controls needed in the requirements as I have outlined in the section on how the equipment is controlled, it will not address the first requirements which are the basis of monitoring and measurement equipment. These requirements are designed to make sure that the equipment is used in such a way that it will capture not only accurate, but relevant and adequate data. All of this is important, because capturing data that is not relevant and adequate is a waste of time and money, since this will not help with making good evidence-based decisions to improve your company processes and product. After all, this is why you implemented an ISO 9001 quality management system in the first place.
Click here to see a free preview of the Procedure for Equipment Maintenance and Measurement Equipment template.