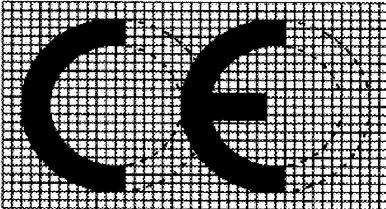
- The CE marking shall consist of the initials ‘CE’ taking the following form:
- If the CE marking is reduced or enlarged, the proportions given in the above graduated drawing shall be respected.
- The various components of the CE marking shall have substantially the same vertical dimension, which may not be less than 5 mm. This minimum dimension may be waived for small-scale devices.