ISO 9001
English
Implementation, maintenance, training, and knowledge products for Information Security Management Systems (ISMS) according to the ISO 27001 standard.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
All required policies, procedures, and forms to implement an ISMS according to ISO 27001.
Train your key people about ISO 27001 requirements and provide cybersecurity awareness training to all of your employees.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Compliance and training products for critical infrastructure organizations for the European Union’s Network and Information Systems cybersecurity directive.
All required policies, procedures, and forms to comply with the NIS 2 cybersecurity directive.
Company-wide training program for employees and senior management to comply with Article 20 of the NIS 2 cybersecurity directive.
Compliance and training products for financial entities for the European Union’s DORA regulation.
All required policies, procedures, and forms to comply with the DORA regulation.
Company-wide cybersecurity and resilience training program for all employees, to train them and raise awareness about ICT risk management.
Accredited courses for individuals and DORA professionals who want the highest-quality training and certification.
Compliance and training products for personal data protection according to the European Union’s General Data Protection Regulation.
All required policies, procedures, and forms to comply with the EU GDPR privacy regulation.
Train your key people about GDPR requirements to ensure awareness of data protection principles, privacy rights, and regulatory compliance.
Accredited courses for individuals and privacy professionals who want the highest-quality training and certification.
Implementation, training, and knowledge products for Quality Management Systems (QMS) according to the ISO 9001 standard.
All required policies, procedures, and forms to implement a QMS according to ISO 9001.
Accredited courses for individuals and quality professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 and the QMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for Environmental Management Systems (EMS) according to the ISO 14001 standard.
All required policies, procedures, and forms to implement an EMS according to ISO 14001.
Accredited courses for individuals and environmental professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 14001 and the EMS using Advisera’s proprietary AI-powered knowledge base.
Implementation and training products for Occupational Health & Safety Management Systems (OHSMS) according to the ISO 45001 standard.
All required policies, procedures, and forms to implement an OHSMS according to ISO 45001.
Accredited courses for individuals and health & safety professionals who want the highest-quality training and certification.
Implementation and training products for medical device Quality Management Systems (QMS) according to the ISO 13485 standard.
All required policies, procedures, and forms to implement a medical device QMS according to ISO 13485.
Accredited courses for individuals and medical device professionals who want the highest-quality training and certification.
Compliance products for the European Union’s Medical Device Regulation.
All required policies, procedures, and forms to comply with the EU MDR.
Implementation products for Information Technology Service Management Systems (ITSMS) according to the ISO 20000 standard.
All required policies, procedures, and forms to implement an ITSMS according to ISO 20000.
Implementation products for Business Continuity Management Systems (BCMS) according to the ISO 22301 standard.
All required policies, procedures, and forms to implement a BCMS according to ISO 22301.
Implementation products for testing and calibration laboratories according to the ISO 17025 standard.
All required policies, procedures, and forms to implement ISO 17025 in a laboratory.
Implementation products for automotive Quality Management Systems (QMS) according to the IATF 16949 standard.
All required policies, procedures, and forms to implement an automotive QMS according to IATF 16949.
Implementation products for aerospace Quality Management Systems (QMS) according to the AS9100 standard.
All required policies, procedures, and forms to implement an aerospace QMS according to AS9100.
Implementation, maintenance, training, and knowledge products for consultancies.
Handle multiple ISO 27001 projects by automating repetitive tasks during ISMS implementation.
All required policies, procedures, and forms to implement various standards and regulations for your clients.
Grow your business by organizing cybersecurity and compliance training for your clients under your own brand using Advisera’s learning management system platform.
Accredited Lead Auditor and Lead Implementer courses for ISO standards and DORA, and an advanced course to help consultants grow their business.
Get instant answers to any questions related to ISO 27001 (ISMS), ISO 9001 (QMS), and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Find new clients, potential partners, and collaborators and meet a community of like-minded professionals locally and globally.
Implementation, maintenance, training, and knowledge products for the IT industry.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
Documentation to comply with ISO 27001 (cybersecurity), ISO 22301 (business continuity), ISO 20000 (IT service management), GDPR (privacy), NIS 2 (critical infrastructure cybersecurity), and DORA (cybersecurity for financial sector).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Compliance, training, and knowledge products for essential and important organizations.
Documentation to comply with NIS 2 (cybersecurity), GDPR (privacy), ISO 27001 (cybersecurity), and ISO 22301 (business continuity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for manufacturing companies.
Documentation to comply with ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for transportation & distribution companies.
Documentation to comply with ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for schools, universities, and other educational organizations.
Documentation to comply with ISO 27001 (cybersecurity), ISO 9001 (quality), and GDPR (privacy).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS) and ISO 9001 (QMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, maintenance, training, and knowledge products for telecoms.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
Documentation to comply with ISO 27001 (cybersecurity), ISO 22301 (business continuity), ISO 20000 (IT service management), GDPR (privacy), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, maintenance, training, and knowledge products for banks, insurance companies, and other financial organizations.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
Documentation to comply with DORA (cybersecurity for financial sector), ISO 27001 (cybersecurity), ISO 22301 (business continuity), and GDPR (privacy).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for local, regional, and national government entities.
Documentation to comply with ISO 27001 (cybersecurity), ISO 9001 (quality), GDPR (privacy), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS) and ISO 9001 (QMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for hospitals and other health organizations.
Documentation to comply with ISO 27001 (cybersecurity), ISO 9001 (quality), ISO 14001 (environmental), ISO 45001 (health & safety), NIS 2 (critical infrastructure cybersecurity) and GDPR (privacy).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS), ISO 9001 (QMS), and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for the medical device industry.
Documentation to comply with MDR and ISO 13485 (medical device), ISO 27001 (cybersecurity), ISO 9001 (quality), ISO 14001 (environmental), ISO 45001 (health & safety), NIS 2 (critical infrastructure cybersecurity) and GDPR (privacy).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS), ISO 9001 (QMS), and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for the aerospace industry.
Documentation to comply with AS9100 (aerospace), ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for the automotive industry.
Documentation to comply with IATF 16949 (automotive), ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for laboratories.
Documentation to comply with ISO 17025 (testing and calibration laboratories), ISO 9001 (quality), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and quality professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 and the QMS using Advisera’s proprietary AI-powered knowledge base.
Implement ISO 9001 yourself, and do it easily and efficiently with our Documentation Toolkit.
76 document templates – unlimited access to all documents required for ISO 9001 certification, plus commonly used non-mandatory document
Editable MS Word and MS Excel policies, procedures, plans, and forms that you can adapt to your company needs.
Access to video tutorials
Video that help you fill out the most important documents using real data – what you need to keep, what you can change, and what you can delete.
Email support
We will answer your questions within 1 business day. You can send up to 10 questions per month.
Expert review of a document
After completing the document, you can send it for our review, and we’ll give you our comments on what you need to improve to make it compliant with the standards.
One hour of live one-on-one online consultations with an ISO 9001 expert
Our expert will speak to you via Zoom, MS Teams, or telephone, at a time that’s convenient for you, where you can discuss how to resolve any issues you face in the implementation; the expert will also provide tips on the next steps in your project.
Fully optimized for
small and medium-sized companies
Look at EVERY template in the ISO 9001 Premium Documentation Toolkit – for free! – before making a purchase decision.
PRODUCTION & SERVICE PROVISION
POLICY AND MANUAL
DOCUMENT CONTROL
CONTEXT AND INTERESTED PARTIES
HUMAN RESOURCES
RISKS AND OPORTUNITIES
SALES PROCEDURE
DESIGN & DEVELOPMENT
PURCHASING PROCEDURE
WAREHOUSING PROCEDURE
NONCONFORMITIES
EQUIPMENT MAINTENANCE
CUSTOMER SATISFACTION
INTERNAL AUDIT
MANAGEMENT REVIEW

Advisera’s toolkits are developed by some of the most experienced auditors, trainers and consultants for the ISO 9001 standard.

All documents are 80% pre-written. By filling in the specifics of your company, you’ll save both time and money with your ISO 9001 implementation process.
We have built the toolkit to help small businesses minimize the time and cost of implementation. Our easy-to-use toolkit will help you implement your quality management policies and set yourself up for ISO 9001 certification.
We’ve done 80% of the work a consultant would charge you for. Anything that can be prefilled in the documents is already done, and the remaining adaptation you need to do is clearly marked with comments and instructions.
The toolkit documents are organized to guide you on your implementation path. They’re structured in clearly numbered folders, so that you know where to start, and – after each document is completed – where to go next.
Simply move through the documents, filling in the specifics for your company as instructed. Our experts have even added some instructions on what to enter, to help you move through the implementation as efficiently as possible.
Completing some parts of a document might be a challenge for you if you’ve never done this before. In these cases, we’ve added detailed instructions and, where needed, links to articles and video tutorials that will help you understand and complete these sections.
Most companies have a specific design and structure for their official documents. There’s header information, confidentiality level, even prescribed graphic design and fonts. All of our documents are fully customizable, so that you can make them look just the way they should.
ISO 9001 certification is much more than just documentation. The implementation of the standard needs to be appropriate to your company, and you need to deal with your employees, your management, and your existing processes in an appropriate way.
This is why our experts are on hand with live online support to answer any difficult questions – we can set up a call via Zoom, MS Teams, or through any other method convenient for you; or, we can answer your questions via email – whatever suits you best.

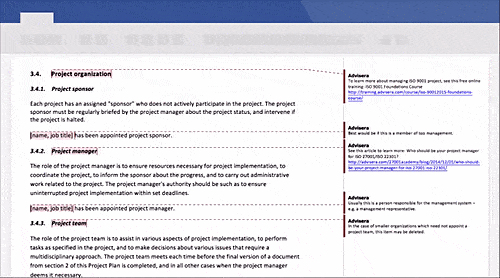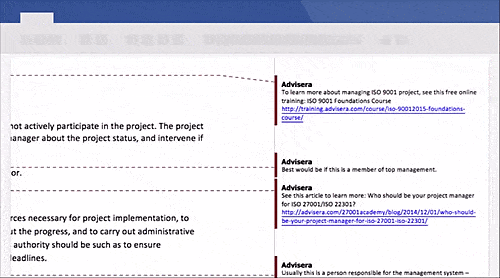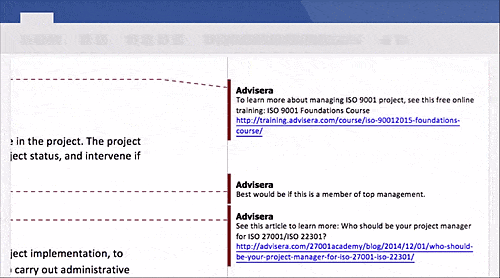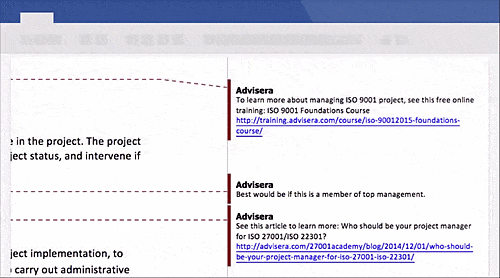
Schedule a free presentation, and our representative will show you any document you're interested in.
Our ISO 9001 expert will meet with you regularly – he will tell you where to start, what the next steps are, and how to resolve any issues you may face. You can meet via Zoom, MS Teams, or through any other means at your convenience.
Reach out to us at any time during your implementation project with unlimited email support, and have your questions answered within 24 hours by our experts.
Once you complete your documents, let our experts review them – they’ll provide you with feedback and indicate what needs to be improved.
Our team includes some of the most experienced auditors, trainers and consultants for the ISO 9001 standard. In addition, we pride ourselves on the communication skills of our expert team, which helps us to establish stable and personal relationships with our clients.



1 hour call where we can check the most important items that the certification auditor will be looking for
1 hour call where we can check the most important items that the certification auditor will be looking for
1 hour call where we can check the most important items that the certification auditor will be looking for
1 hour call where we can check the most important items that the certification auditor will be looking for
Immediately after the transaction is processed, you will receive an email with a download link. It could not be quicker or simpler.
We take all major credit cards, PayPal payment, and we can accept a wire transfer from your bank account.
We proudly use Secure Socket Layer (SSL) technology, which is the industry standard. This technology encrypts your credit card information, keeping it secure, and sends it directly to the payment processor. We never store – or even see – your payment information.
We gladly accept more than 50 commonly used currencies, including the US Dollar, the Euro, the British Pound, and the Swiss Franc.
Please do! Click on the "DOWNLOAD FREE TOOLKIT DEMO" button and enter your name and your email address. You can instantly access a free preview of each document template, helping you make up your mind. This is a great chance to see how each document looks, and how easy they are to complete.
Yes. Also, you are entitled to receive free updates of those toolkits for one year after your purchase date.
Advisera Expert Solutions Ltd is a company specialized in providing online support for ISO implementation. In the last 10 years it covers all the major ISO standards, and is selling its products in more than 100 countries worldwide. Read more here.
Schedule a free presentation, and our representative will show you any document you're interested in.
Provides a structured how-to guide how improve QMS documentation.
Andre van Hees
Well there are years of work, which thanks to the toolkit, can be used to polish the QMS.
Vicente Barahona
Other than just documentation, I like the blogs, e-seminars, specialist's comments, and articles so it helps me to understand the technicality of the procedure before I modify the document as per my needs.
Rajesh Bharadwaj






Get a FREE preview of the ISO 9001 Premium Documentation Toolkit. Once you download the free demo, our representative will contact you and show you any document you’re interested in.
