Waqas Imam
Waqas Imam
September 21, 2017
Implementation, maintenance, training, and knowledge products for Information Security Management Systems (ISMS) according to the ISO 27001 standard.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
All required policies, procedures, and forms to implement an ISMS according to ISO 27001.
Train your key people about ISO 27001 requirements and provide cybersecurity awareness training to all of your employees.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Compliance and training products for critical infrastructure organizations for the European Union’s Network and Information Systems cybersecurity directive.
All required policies, procedures, and forms to comply with the NIS 2 cybersecurity directive.
Company-wide training program for employees and senior management to comply with Article 20 of the NIS 2 cybersecurity directive.
Compliance and training products for financial entities for the European Union’s DORA regulation.
All required policies, procedures, and forms to comply with the DORA regulation.
Company-wide cybersecurity and resilience training program for all employees, to train them and raise awareness about ICT risk management.
Accredited courses for individuals and DORA professionals who want the highest-quality training and certification.
Training products for Artificial Intelligence Management Systems (AIMS) and AI governance according to the ISO 42001 standard.
Accredited courses for individuals, consultants, and AI professionals who want the highest-quality training and certification in AI governance and compliance.
Train your key people on ISO 42001 requirements and provide company-wide AI governance training so employees learn how to use AI responsibly and in compliance with your policies.
Compliance and training products for personal data protection according to the European Union’s General Data Protection Regulation.
All required policies, procedures, and forms to comply with the EU GDPR privacy regulation.
Train your key people about GDPR requirements to ensure awareness of data protection principles, privacy rights, and regulatory compliance.
Accredited courses for individuals and privacy professionals who want the highest-quality training and certification.
Implementation, training, and knowledge products for Quality Management Systems (QMS) according to the ISO 9001 standard.
All required policies, procedures, and forms to implement a QMS according to ISO 9001.
Accredited courses for individuals and quality professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 and the QMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for Environmental Management Systems (EMS) according to the ISO 14001 standard.
All required policies, procedures, and forms to implement an EMS according to ISO 14001.
Accredited courses for individuals and environmental professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 14001 and the EMS using Advisera’s proprietary AI-powered knowledge base.
Implementation and training products for Occupational Health & Safety Management Systems (OHSMS) according to the ISO 45001 standard.
All required policies, procedures, and forms to implement an OHSMS according to ISO 45001.
Accredited courses for individuals and health & safety professionals who want the highest-quality training and certification.
Implementation and training products for medical device Quality Management Systems (QMS) according to the ISO 13485 standard.
All required policies, procedures, and forms to implement a medical device QMS according to ISO 13485.
Accredited courses for individuals and medical device professionals who want the highest-quality training and certification.
Compliance products for the European Union’s Medical Device Regulation.
All required policies, procedures, and forms to comply with the EU MDR.
Implementation products for Information Technology Service Management Systems (ITSMS) according to the ISO 20000 standard.
All required policies, procedures, and forms to implement an ITSMS according to ISO 20000.
Implementation products for Business Continuity Management Systems (BCMS) according to the ISO 22301 standard.
All required policies, procedures, and forms to implement a BCMS according to ISO 22301.
Implementation products for testing and calibration laboratories according to the ISO 17025 standard.
All required policies, procedures, and forms to implement ISO 17025 in a laboratory.
Implementation products for automotive Quality Management Systems (QMS) according to the IATF 16949 standard.
All required policies, procedures, and forms to implement an automotive QMS according to IATF 16949.
Implementation products for aerospace Quality Management Systems (QMS) according to the AS9100 standard.
All required policies, procedures, and forms to implement an aerospace QMS according to AS9100.
Implementation, maintenance, training, and knowledge products for consultancies.
Handle multiple ISO 27001 projects by automating repetitive tasks during ISMS implementation.
All required policies, procedures, and forms to implement various standards and regulations for your clients.
Grow your business by organizing cybersecurity and compliance training for your clients under your own brand using Advisera’s learning management system platform.
Accredited Lead Auditor and Lead Implementer courses for ISO standards and DORA, and an advanced course to help consultants grow their business.
Get instant answers to any questions related to ISO 27001 (ISMS), ISO 9001 (QMS), and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Find new clients, potential partners, and collaborators and meet a community of like-minded professionals locally and globally.
Implementation, maintenance, training, and knowledge products for the IT industry.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
Documentation to comply with ISO 27001 (cybersecurity), ISO 22301 (business continuity), ISO 20000 (IT service management), GDPR (privacy), NIS 2 (critical infrastructure cybersecurity), and DORA (cybersecurity for financial sector).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity, privacy, and AI program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Compliance, training, and knowledge products for essential and important organizations.
Documentation to comply with NIS 2 (cybersecurity), GDPR (privacy), ISO 27001 (cybersecurity), and ISO 22301 (business continuity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for manufacturing companies.
Documentation to comply with ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for transportation & distribution companies.
Documentation to comply with ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for schools, universities, and other educational organizations.
Documentation to comply with ISO 27001 (cybersecurity), ISO 9001 (quality), and GDPR (privacy).
Company-wide cybersecurity and AI governance awareness program for all employees, to decrease incidents, support a successful cybersecurity program, and ensure responsible use of AI.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS) and ISO 9001 (QMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, maintenance, training, and knowledge products for telecoms.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
Documentation to comply with ISO 27001 (cybersecurity), ISO 22301 (business continuity), ISO 20000 (IT service management), GDPR (privacy), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, maintenance, training, and knowledge products for banks, insurance companies, and other financial organizations.
Automate your ISMS implementation and maintenance with the Risk Register, Statement of Applicability, and wizards for all required documents.
Documentation to comply with DORA (cybersecurity for financial sector), ISO 27001 (cybersecurity), ISO 22301 (business continuity), and GDPR (privacy).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity, privacy, and AI program.
Accredited courses for individuals and security professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 and the ISMS using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for local, regional, and national government entities.
Documentation to comply with ISO 27001 (cybersecurity), ISO 9001 (quality), GDPR (privacy), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity, privacy, and AI program.
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity, privacy, and AI program.
Get instant answers to any questions related to ISO 27001 (ISMS) and ISO 9001 (QMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for hospitals and other health organizations.
Documentation to comply with ISO 27001 (cybersecurity), ISO 9001 (quality), ISO 14001 (environmental), ISO 45001 (health & safety), NIS 2 (critical infrastructure cybersecurity) and GDPR (privacy).
Company-wide cybersecurity and AI governance awareness program for all employees, to decrease incidents, support a successful cybersecurity program, and ensure responsible use of AI.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS), ISO 9001 (QMS), and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for the medical device industry.
Documentation to comply with MDR and ISO 13485 (medical device), ISO 27001 (cybersecurity), ISO 9001 (quality), ISO 14001 (environmental), ISO 45001 (health & safety), NIS 2 (critical infrastructure cybersecurity) and GDPR (privacy).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity, privacy, and AI program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 27001 (ISMS), ISO 9001 (QMS), and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for the aerospace industry.
Documentation to comply with AS9100 (aerospace), ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for the automotive industry.
Documentation to comply with IATF 16949 (automotive), ISO 9001 (quality), ISO 14001 (environmental), and ISO 45001 (health & safety), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity and AI program.
Accredited courses for individuals and professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 (QMS) and ISO 14001 (EMS) using Advisera’s proprietary AI-powered knowledge base.
Implementation, training, and knowledge products for laboratories.
Documentation to comply with ISO 17025 (testing and calibration laboratories), ISO 9001 (quality), and NIS 2 (critical infrastructure cybersecurity).
Company-wide cybersecurity awareness program for all employees, to decrease incidents and support a successful cybersecurity program.
Accredited courses for individuals and quality professionals who want the highest-quality training and certification.
Get instant answers to any questions related to ISO 9001 and the QMS using Advisera’s proprietary AI-powered knowledge base.
 Waqas Imam
Waqas Imam
A patient undergoing a surgical procedure places his trust in the surgeon, the institution, and the procedure of surgery. He is least concerned about the medical devices, and not aware of the associated safety risks. Therefore, the patient accepts the risks of a medical device without any knowledge or awareness. This is the reason that medical device manufacturers must ensure that their product is safe with the help of a robust risk management process.
ISO 13485 references ISO 14971:2007 (Medical devices – Application of risk management to medical devices) for risk management. ISO 13485 defines risk based on ISO 14971 as “the combination of the probability of occurrence of harm and the severity of that harm.”
The process flow for risk management based on ISO 14971 is shown in figure 1. According to clause 3 in ISO 14971, top management must:
As with other management standards, people who perform risk assessment should be competent and knowledgeable (e.g., through trainings on ISO 14971, medical device application, etc.).
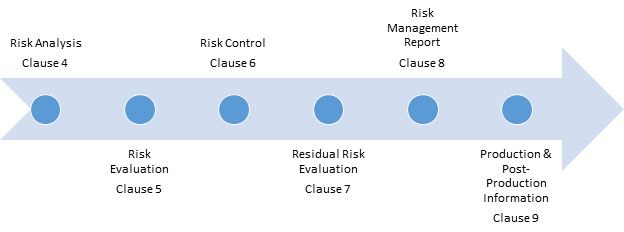 Figure 1. Risk management process flow
Figure 1. Risk management process flow
Another important element in risk management (to ensure traceability) is a risk management file, which is established for every medical device. The file is used to keep record of:
The risk management file will be used to gather all information related to risk, even in post-production situations.
The process of risk management has the following steps:
1) Risk analysis – Risk analysis is performed on each medical device, and possible hazards are identified. Risk is estimated for each hazardous situation. Characteristics that can foreseeably affect the safety of the medical device are also listed. Risk analysis should also incorporate a combination of hazardous events that can result in a hazardous situation, whereas reasonably foreseeable combinations of such events should be analyzed separately. For example, when a heel stick is used to collect blood from infants for testing, the blood is warmed with a chemical pack. The sudden rupturing of this chemical pack is a foreseeable effect of the characteristics of the chemical pack, and the hazardous event is a combination of the heel stick used for collecting the sample (likely a negligible hazard) and the chemical pad used to ease the process of sampling. The risk management file is updated accordingly based on all analysis results.
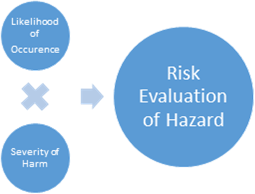
2) Risk evaluation – Each hazardous situation is studied, and then the organization’s risk acceptability criteria are used to confirm whether risk reduction is needed for this hazard or not. The results of risk evaluation activities are also recorded in the file. Risk evaluation is normally done by multiplying the severity of the hazard by the likelihood of its occurrence.
Figure 2. Risk evaluation
3) Risk control – Risk control is a risk reduction process in which an unacceptable risk is minimized. The effectiveness of the control is measured by reevaluation of residual risk, i.e., remaining risk after the control is implemented. Sometimes, controls allocated to minimize a risk add another risk hazard – such controls are ineffective until, and unless, the new risks are within acceptable range or controlled within acceptable limits. A risk control is chosen from the available options based on the following factors:
When implemented, risk controls are verified. If the residual risk is unacceptable, a risk benefit analysis is conducted. If an additional control is impractical, then the risk benefit analysis should dictate whether the medical benefits of the device outweigh the residual risk. Records of each step of risk control are maintained in the risk management file, which includes control options, selection of control, risk control review, control verification, residual risk calculation, risk benefit analysis, etc.
4) Residual risk evaluation – Residual risk evaluation is done after all controls are in place and effective. A file is maintained with the risk management register after all risks have been properly controlled, and records are maintained. Any change may require reevaluation of overall residual risks.
5) Risk management report – Just as management reviews are planned for the Quality Management System, likewise, such reviews should be planned for the risk management system. Before a medical device enters the commercial market, a review should be conducted. Based on the review, a risk management report is prepared. The report should include the results of the review and be incorporated into the risk management file.
6) Information from production and post-production – A system for monitoring the performance of the medical device should be developed, established, and maintained. The results should be recorded in the risk management file. Information that comes from production includes any defects or failures in clinical trials, and results of post-production include any customer complaints or product failures that may increase the risk (because of increased likelihood of occurrence).
With the help of a risk management system based on ISO 13485 and ISO 14971, each phase of a risk management cycle is documented comprehensively to demonstrate the manufacturer’s commitment to controlling risk in the life of the medical device. A strong risk management system also provides significant value by helping with the development, manufacture, and delivery of new medical devices. Devices under development are subject to higher levels of scrutiny. Also, a risk management system helps with documenting modifications to ensure product safety, functionality, and usability.
For reusable medical devices, a robust risk management evaluation will also classify risks related to product reapplication and reprocess. Additional benefits of such a system allow for faster market penetration and better competitive standing. A great motivational factor, isn’t it?
To comply with all ISO 13485 requirements, use this helpful ISO 13485 Documentation Toolkit that provides QMS documents for medical device companies.

You may unsubscribe at any time. For more information, please see our privacy notice.