Updated: July 2, 2024.
Usually, when people think of Quality Management System (QMS) documentation, they envision loads of documents and unnecessary bureaucratic procedures. This is because companies often go overboard when documenting their Quality Management Systems. So, how do you structure ISO 9001 QMS documentation without creating excess overhead? What is the QMS documentation structure that fits your company style? Learn in this article.
- Quality Policy
- Quality Manual
- Procedures
- Work Instructions
- Records and Forms
It is true that the international standard for Quality Management Systems (ISO 9001) requires certain documentation (See this article: ISO 9001 Documentation Requirements – The complete list). The purpose and the benefits of the QMS document structure are manifold: It provides a clear framework of the operations in an organization, it allows consistency of processes and better understanding of the QMS, and it ensures evidence for the achievement of objectives and goals. When designing your QMS documentation structure, you should focus on efficiency and create processes and documents that are applicable in your organization.
QMS documentation structure and hierarchy
The QMS structure and QMS documentation hierarchy help organize documents in a meaningful way. So, how many levels of documentation are there in the QMS documentation hierarchy? On the top of the QMS hierarchy is the Quality Policy, followed by the Quality Manual, procedures, work instructions, quality plans, and records. The QMS documentation hierarchy is presented in the diagram below:
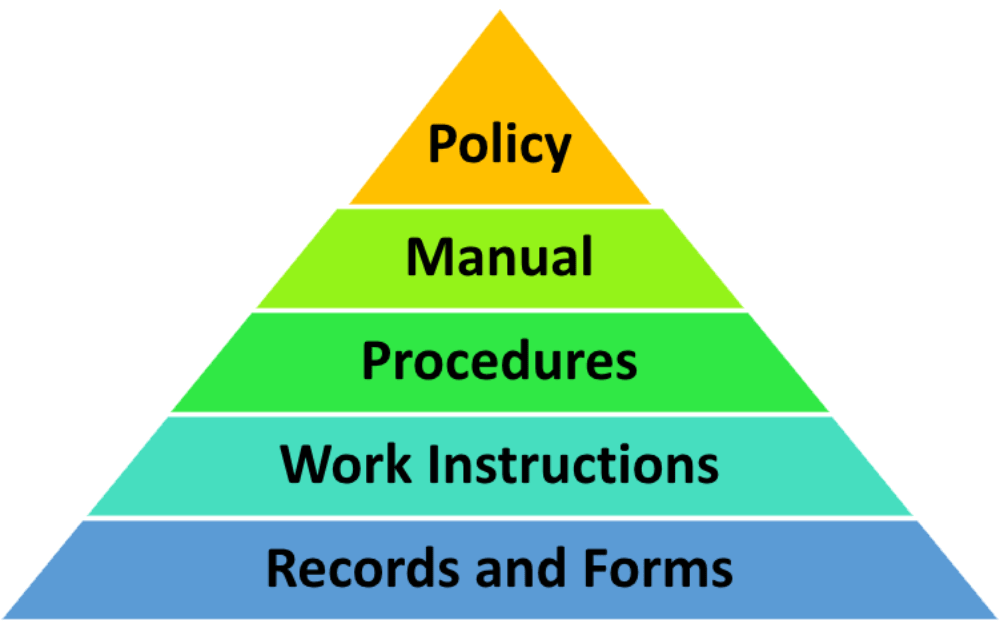
ISO 9001 requires different types of documents; however, they do not need to be written as separate documents. The standard is flexible, so that the organization can decide on the size of the documents and the level of details documented. For example, some small or micro companies do not have formal system documents like procedures and work instructions. They define their instructions within a Quality Manual, and they only create certain documents and records, like the Quality Policy, context of the organization, competencies, internal audit, management review, and performance evaluation.
How do you structure Quality Management System documentation?
The international standard ISO 10013:2021 Quality management systems – Guidelines for documented information gives directions for an effective QMS documentation numbering system, as well as an overview of recommended contents and structures of the different QMS document types. The following recommendations take into consideration the ISO 10013 guidelines.
1) Quality Policy. A policy represents a declarative statement by an organization — it should state the commitment of the organization to deliver quality products or services to its customers, as well as to continual improvement. Usually, this policy is used for promotional purposes and should be displayed on the organization’s premises and posted on websites, so a clear and short Quality Policy is convenient and is the general practice.
The Quality Policy defines the quality objectives to which the organization strives. The quality goals of organizations are defined by quantifying the quality objectives.
2) Quality Manual. The Quality Manual is a document that outlines the structure of an organization’s QMS and provides information about the organization’s commitment to quality. It should fit your organization. The structure and the content of the manual can vary depending on the size of the organization, the complexity of its operations, and the competence of the personnel. Small organizations can document the entire QMS in one manual. On the other hand, large international organizations may have several different quality manuals. Generally, the manual includes the QMS scope, exclusions from the standard, references to relevant documents, and the business process model. The Quality Policy and the objectives can be part of the manual as well.
The Quality Manual should include most of the following elements: title and table of contents; scope of the QMS; exclusions from ISO 9001, versioning information, and approval; Quality Policy and objectives; QMS description, the business process model of the organization; definition of responsibilities for all personnel; and references to relevant documents and relevant appendices. More information on how to document an effective Quality Manual can be found in this article: How to write a short, concise Quality Manual.
3) Quality procedures. Quality procedures are documented instructions that outline the steps and activities to be followed in the performance of specific processes within a Quality Management System. The quality procedures can have different formats and structures. They can be narrative, i.e., described through text; they can be more structured by using tables; they can be more illustrative, i.e., through flow charts; they can be even descriptive imagery, i.e. photos; or they can be any combination of the above.
Quality procedures should include the following elements:
- Title – for identification of the procedure.
- Purpose – describing the rationale behind the procedure.
- Scope – to explain what aspects will be covered in the procedure, and which aspects will not be covered.
- Responsibilities and authorities of all people/functions included in any part of the procedure.
- Definitions and lists of all records that result from the activities described in the procedure.
- Document control – identification of changes, date of review, and approval and version of the document should be included in accordance with the established practice for document control.
- Description of activities – this is the main section of the procedure; it relates all the other elements of the procedure and describes what should be done, by whom and how, when and where. In some cases, “why” should be clarified as well. Additionally, the inputs and the outputs of the activities should be explained, including the required resources.
- Appendices may be included, if needed.
4) Work instructions. Work instructions can be part of a procedure, or they can be referenced from a procedure. Generally, work instructions have a similar structure to the procedures and cover the same elements; however, the work instructions include details of activities that need to be realized, focusing on the sequencing of the steps, tools, and methods to be used and required accuracy.
Training of personnel and the use of competent personnel can decrease the need for highly detailed work instructions. More detail on this topic can be found in Using Competence, Training and Awareness to Replace Documentation in your QMS.
5) Records and forms. In order to demonstrate that your processes met the requirements, you will want to keep some evidence; this is where forms and records are used. A record is what has been chosen by the process owner to demonstrate that the process and activities have been conducted in the way prescribed in the procedures and work instructions. Forms are the blank templates to be filled in with information that will become these records. Make your records and forms practical by being concise and simply recording the information required; do not make employees write essays to complete the form and create the record. Keep in mind that both records and forms play a crucial role in the implementation and maintenance of the QMS.
Good QMS documentation is essential for an effective Quality Management System
Dimensioning the QMS documentation based on your organizational needs is essential for a functional QMS. Moreover, proper QMS document management will make your operations much easier, while incorrect documentation will bring you nothing but trouble.
Download a free preview of the ISO 9001 Premium Documentation Toolkit to see the structure for each document mentioned above.
Contributor
Kristina Bombas Georgievska, Lead Auditor for an accredited certification body for management systems. Experience with implementation of management standards for nearly 20 years and implementation of specific and technical standards for conformity assessment (laboratory for testing; certification of products, processes, and services) in the civil engineering/construction sector as well as medical devices. Expertise in quality infrastructure and ISO 9001, ISO 14001, ISO 45001, ISO 13485, ISO/IEC 17025, ISO/IEC 17065, ISO/IEC 27001, CPR 305/2017, and MDR 2017/745.

 Ana Meskovska
Ana Meskovska 



