Updated: July 7, 2023.
Version 2015 of the ISO 9001 standard has brought some changes, so it’s important to know which documents are mandatory in this revision. How many documents are required in the QMS documents list?
So, here is the list of ISO 9001 documentation requirements – below, you will see not only ISO 9001 mandatory documents, but also the most commonly used documents for ISO 9001 implementation.
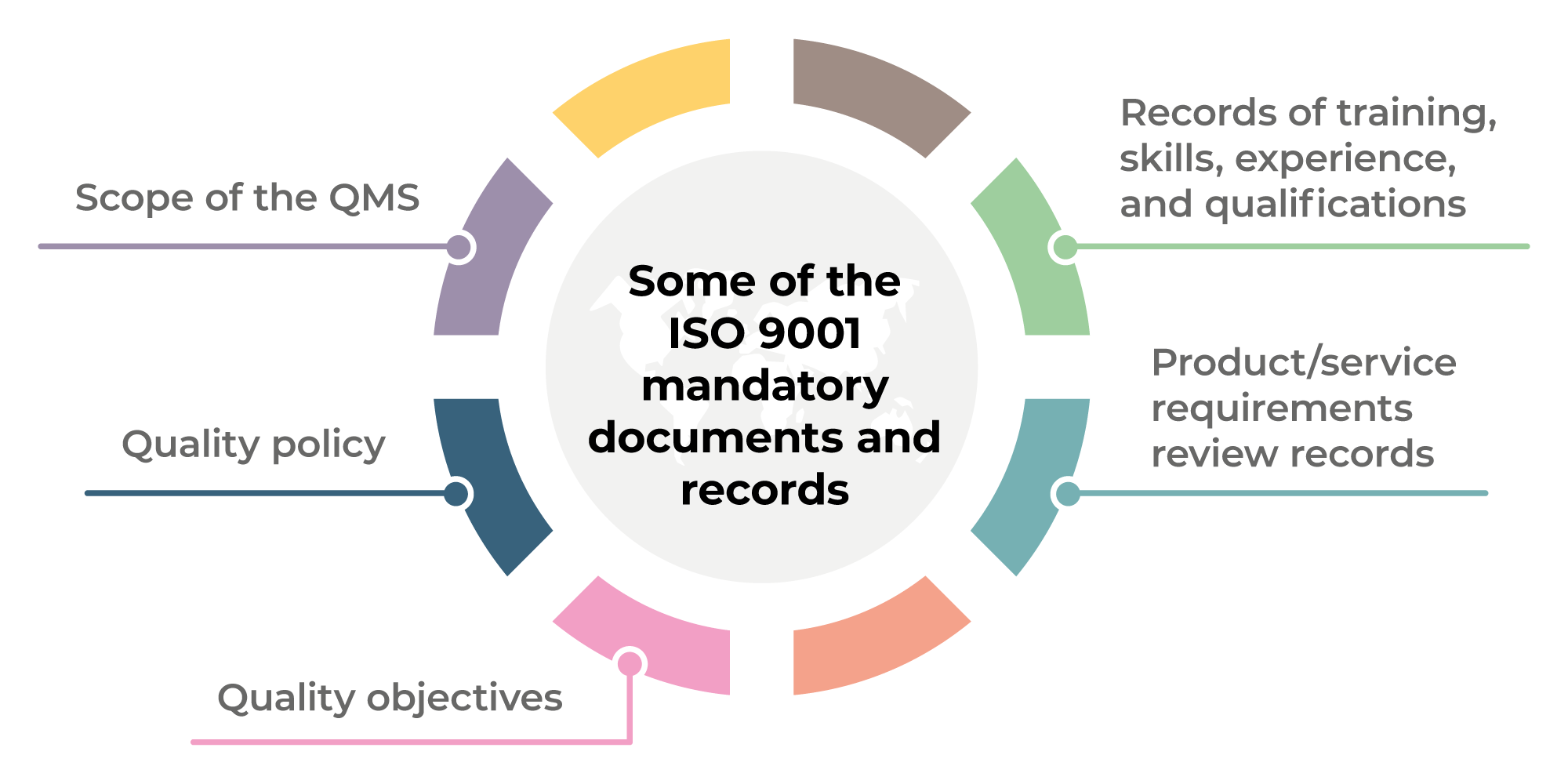
- Scope of the QMS
- Quality policy
- Quality objectives
- Records of training, skills, experience, and qualifications
- Product/service requirements review records
ISO 9001 mandatory documents and records
Here is the quality documents list you need to produce if you want to be compliant with ISO 9001:2015. (Please note that some of the documents will not be mandatory if the company does not perform relevant processes.)
- Scope of the QMS (clause 4.3)
- Quality policy (clause 5.2)
- Quality objectives (clause 6.2)
- Criteria for evaluation and selection of suppliers (clause 8.4.1)
Besides this quality documentation list, here are the mandatory records:
*Note that records marked with * are only mandatory in cases when the relevant clause is not excluded
Non-mandatory documents
There are numerous non-mandatory documents that can be used for ISO 9001 implementation. However, these non-mandatory documents are the most commonly used:
| What must be documented | ISO 9001 reference |
| Procedure for determining context of the organization and interested parties | clauses 4.1 and 4.2 |
| Procedure for addressing risks and opportunities | clause 6.1 |
| Procedure for competence, training and awareness | clauses 7.1.2, 7.2 and 7.3 |
| Procedure for equipment maintenance and measuring equipment | clause 7.1.5 |
| Procedure for document and record control | clause 7.5 |
| Sales procedure | clause 8.2 |
| Procedure for design and development | clause 8.3 |
| Procedure for production and service provision | clause 8.5 |
| Warehousing procedure | clause 8.5.4 |
| Procedure for management of nonconformities and corrective actions | clauses 8.7 and 10.2 |
| Procedure for monitoring customer satisfaction | clause 9.1.2 |
| Procedure for internal audit | clause 9.2 |
| Procedure for management review | clause 9.3 |
So, to resume, we have listed the most frequently asked questions about ISO 9001 documentation requirements below.
What are the six documents required by ISO 9001?
While the previous version of ISO 9001 included six required procedures, ISO 9001:2015 does not have any required procedures. The organization needs to determine which procedures are needed within the QMS scope to avoid nonconformity in the processes.
What are the mandatory documents and records required by ISO 9001:2015?
The four documents and 18 records listed above are the ISO 9001 mandatory documents required by the standard; however, each can be removed from the list if that requirement is not included in the QMS scope.
What records must be kept to comply with ISO 9001?
The mandatory records for ISO 9001 include the 18 records above, which need to be kept, unless the specific requirement is excluded from the Quality Management System in the scope document.
How many mandatory procedures are there in ISO 9001:2015?
ISO 9001:2015 includes no mandatory procedures in the requirements; however, organizations may need to include them to ensure that processes occur as planned.
Does ISO 9001 require a quality manual?
While a quality manual is not required by ISO 9001:2015, many companies will create one in response to a customer requirement, or as a way to compile the four mandatory documents of the QMS – scope, quality policy, quality objectives, and supplier selection criteria – into one document.
So, this is it – it might seem like a lot to write, but once you do it, you will have all the aspects of quality management in your company covered.
This ISO 9001 Premium Documentation Toolkit helps with structuring and implementing mandatory documents and records.

 Strahinja Stojanovic
Strahinja Stojanovic